This story is updated as WPR learns more about the coronavirus vaccines and answers your questions submitted to WPR’s WHYsconsin.
The news of effective vaccines against the coronavirus has been a bright spot in a pandemic that has killed more than 3.35 million people worldwide and hundreds of thousands in the United States. In Wisconsin, more than 6,900 people have died from COVID-19 so far.
Now that federal regulators have approved three COVID-19 vaccines, states are busy getting shots into arms. In mid-December, health care workers in Wisconsin began receiving shots. Since then, others with high exposure to the virus in their line of work and who are at high risk of severe complications from the virus have been vaccinated. The vaccine became available to the general public age 12 and up May 12.
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
So, what do we know about the vaccines and what distribution looks like in Wisconsin?
When Can I Get A Shot?
Currently eligible populations:
- All individuals age 12 and up are eligible for the COVID-19 vaccine. This group is known as Phase 2.
Whether you can get a vaccination right away depends on demand and supply. Some people eligible earlier than others have waited weeks before being inoculated.
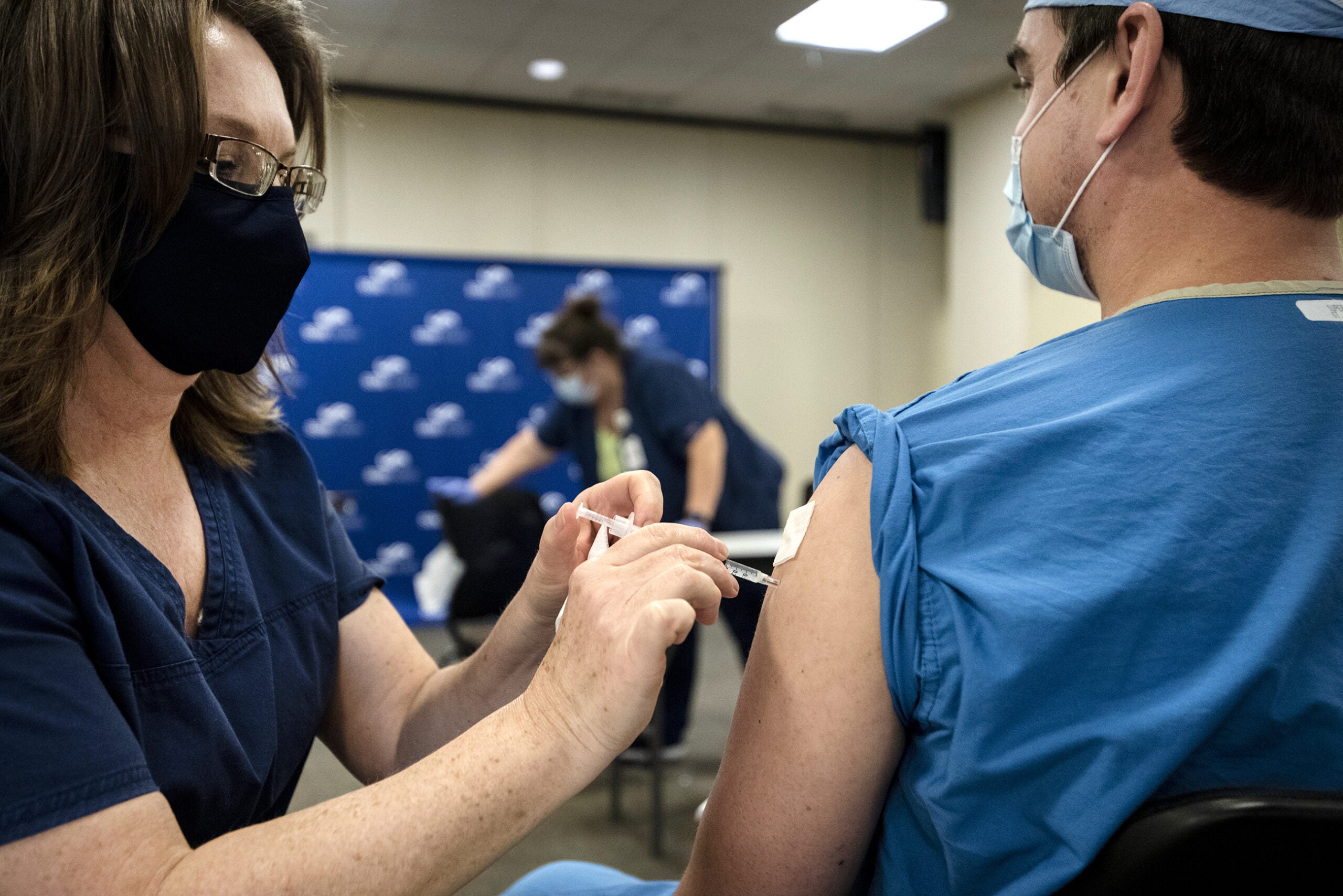
[[{“fid”:”1402071″,”view_mode”:”embed_landscape”,”fields”:{“format”:”embed_landscape”,”alignment”:”right”,”field_image_caption[und][0][value]”:”%3Cp%3EOn%20Monday%2C%20Dec.%2014%2C%202020%2C%20UW%20Health%20respiratory%20therapist%20Tina%20Schubert%2C%20left%2C%20became%20the%20first%20of%20UW%20Health%E2%80%99s%20health%20care%20workers%20to%20receive%20the%20Pfizer%20coronavirus%20vaccine.%20%3Cem%3EPhoto%20courtesy%20of%20UW%20Health.%3C%2Fem%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:”UW Health respiratory therapist Tina Schubert is the first of UW Health’s health care worker to receive the coronavirus vaccine”,”field_file_image_title_text[und][0][value]”:”UW Health respiratory therapist Tina Schubert is the first of UW Health’s health care worker to receive the coronavirus vaccine”},”type”:”media”,”field_deltas”:{“2”:{“format”:”embed_landscape”,”alignment”:”right”,”field_image_caption[und][0][value]”:”%3Cp%3EOn%20Monday%2C%20Dec.%2014%2C%202020%2C%20UW%20Health%20respiratory%20therapist%20Tina%20Schubert%2C%20left%2C%20became%20the%20first%20of%20UW%20Health%E2%80%99s%20health%20care%20workers%20to%20receive%20the%20Pfizer%20coronavirus%20vaccine.%20%3Cem%3EPhoto%20courtesy%20of%20UW%20Health.%3C%2Fem%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:”UW Health respiratory therapist Tina Schubert is the first of UW Health’s health care worker to receive the coronavirus vaccine”,”field_file_image_title_text[und][0][value]”:”UW Health respiratory therapist Tina Schubert is the first of UW Health’s health care worker to receive the coronavirus vaccine”}},”link_text”:false,”attributes”:{“alt”:”UW Health respiratory therapist Tina Schubert is the first of UW Health’s health care worker to receive the coronavirus vaccine”,”title”:”UW Health respiratory therapist Tina Schubert is the first of UW Health’s health care worker to receive the coronavirus vaccine”,”class”:”media-element file-embed-landscape media-wysiwyg-align-right”,”data-delta”:”2″}}]]On May 12, an advisory panel for the Centers for Disease Control and Prevention recommended use of the Pfizer vaccine for children as young as 12 years old. Prior to this, all individuals age 16 and up were eligible for the vaccine as of April 5.
Those age 16 and up with certain pre-existing conditions — Phase 1c — got the go-ahead to get shots starting March 22. Proof of chronic illness was not required by vaccinators, according to the state Department of Health Services.
Teachers and other essential workers, or phase 1b, began to be vaccinated March 1. Frontline health care workers, people in long-term care, first responders, and those age 65 and older were the first to be vaccinated.
Despite a rising number of people being vaccinated, health officials say it’s important to continue wearing masks and limit face-to-face interactions with those outside their immediate family.
“Just because you’ve been vaccinated doesn’t mean you’re surrounded by some magic bubble or force shield that doesn’t allow SARS-CoV-2 to get inside you,” Dr. Mary Beth Graham, an internal medicine infectious disease specialist at the Medical College of Wisconsin, said. “People who are vaccinated and exposed to the virus can still get it in the respiratory tract. It isn’t until it gets in you that the vaccine turns on your immune system to fight it. So you still could potentially pick up the virus and then potentially be infectious for a short period of time while your body’s taking care of it.”
DHS has contracted with Microsoft on an online signup system for vaccination appointments at vaccination sites across the state.
The Wisconsin COVID-19 Vaccine Registry lets people sign up for COVID-19 shots. The registry also sends reminders to those already vaccinated to make sure they get their second dose, if needed, as well as help them monitor for any side effects after they get the vaccine.
Many health systems are scheduling vaccinations for patients through online portals like MyChart. Additionally, pharmacies and local health departments may have their own system to connect vaccinators with those wanting shots. The Wisconsin Vaccine Registry only displays organizations that opt in. DHS says it is not a comprehensive resource for all vaccination options.
What About People With Chronic Health Conditions?
People who are under age 65 with certain chronic health conditions, such as diabetes and obesity, were eligible for vaccinations as of March 22.
DHS Deputy Secretary Julie Willems Van Dijk said those with chronic illnesses do not have to provide their medical records to obtain a vaccine.
“We are not asking people to verify, we are not asking people to have to bring their medical record and prove that they have asthma or diabetes,” she said. “This is not about policing this, this is about creating entry into the vaccine system for people who have these conditions.”
People with chronic health conditions weren’t included in Phase 1b, which included about 600,000 people.
Adults age 65 and older were eligible Jan. 25. There are about 700,000 Wisconsin residents who are 65 and older, according to DHS. As of April 28, 74.4 percent of adults 65 and older were fully vaccinated.
In Wisconsin, close to 90 percent of deaths from COVID-19 have been among people 65 years old or older. The risk of mortality was one of the most important factors the state considered when it determined vaccine order, Willems Van Dijk told WPR.
“But then they also considered risk of exposure, and what groups also posed a risk of … spreading to other people,” Willems Van Dijk said.
Since grocery workers, educators and transit workers all have public-facing jobs that could involve being exposed to and potentially transmitting the virus to others, the state chose to prioritize them.
Another complicating factor is the number of people who could be defined as having a chronic health condition. For example, about one-third of Wisconsin adults are obese, a chronic condition that worsens patients’ outcomes from COVID-19. That’s nearly 1.5 million adults.
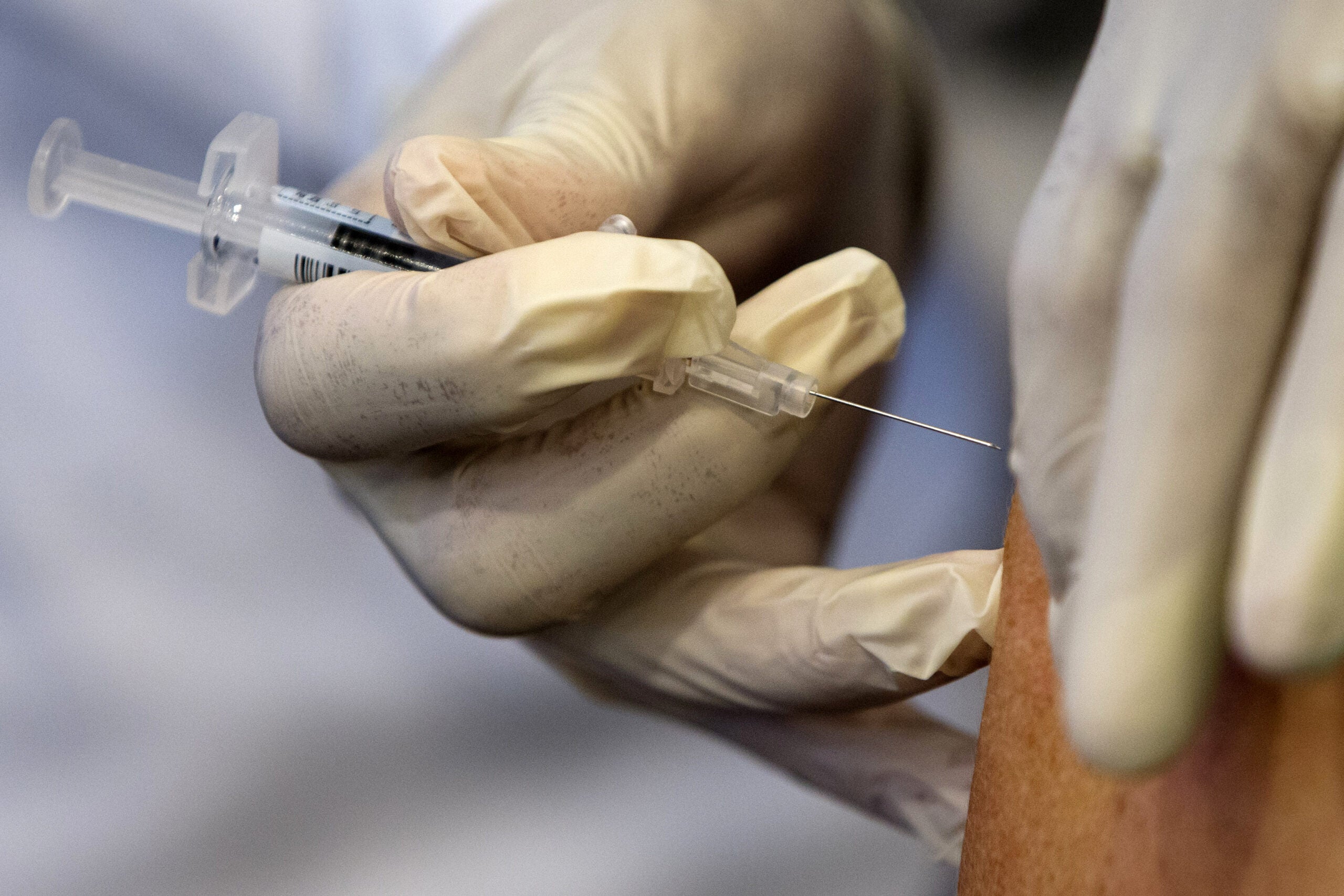
[[{“fid”:”1432411″,”view_mode”:”full_width”,”fields”:{“format”:”full_width”,”alignment”:””,”field_image_caption[und][0][value]”:”%3Cp%3EKevin%20Burns%2C%20a%20student%20nurse%2C%20vaccinates%20Jon%20Ingle%20of%20Palmyra%20on%20Tuesday%2C%20Jan.%2026%2C%202021%2C%20at%20Jefferson%20County%20Fair%20Park.%20%3Cem%3EAngela%20Major%2FWPR%3C%2Fem%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:”a woman in a purple mask has a rolled up sleeve as a student nurse gives her the covid-19 vaccine”,”field_file_image_title_text[und][0][value]”:”Jefferson County COVID-19 Vaccines”},”type”:”media”,”field_deltas”:{“3”:{“format”:”full_width”,”alignment”:””,”field_image_caption[und][0][value]”:”%3Cp%3EKevin%20Burns%2C%20a%20student%20nurse%2C%20vaccinates%20Jon%20Ingle%20of%20Palmyra%20on%20Tuesday%2C%20Jan.%2026%2C%202021%2C%20at%20Jefferson%20County%20Fair%20Park.%20%3Cem%3EAngela%20Major%2FWPR%3C%2Fem%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:”a woman in a purple mask has a rolled up sleeve as a student nurse gives her the covid-19 vaccine”,”field_file_image_title_text[und][0][value]”:”Jefferson County COVID-19 Vaccines”}},”link_text”:false,”attributes”:{“alt”:”a woman in a purple mask has a rolled up sleeve as a student nurse gives her the covid-19 vaccine”,”title”:”Jefferson County COVID-19 Vaccines”,”class”:”media-element file-full-width”,”data-delta”:”3″}}]]
How Many Vaccines Are Available?
While a number of vaccines are under development, only three have been given the green light so far in the U.S.
A big step forward came Dec. 12 when the first vaccine, made by Pfizer, received approval from federal regulators. A week later, the Food and Drug Administration gave the thumbs up for a second vaccine developed by Moderna. On Feb. 27, the FDA approved Johnson & Johnson’s vaccine, which requires one shot for immunization. Pfizer and Moderna’s vaccines require two shots. Because of the urgency, regulators issued an emergency use authorization for the vaccines.
On Tuesday, April 13, state and federal vaccine providers stopped administering administering the J&J vaccine. The Centers for Disease Control and Prevention and the FDA recommended a “pause” in the use of the vaccine to investigate reports of potentially dangerous blood clots. The federal and state agencies said the request is out of an “abundance of caution.”
By not administering the vaccine, the CDC had time to determine if there are other cases of blood clots and had time to give health care providers information on how to treat it, according to DHS. On April 25, the CDC and FDA recommended the use of the J&J vacccine resume.
The first shipment of COVID-19 vaccine made by Pfizer arrived Dec. 14 in Wisconsin. The state’s initial share of Moderna arrived the week of Dec. 21. Wisconsin received its first doses of the J&J vaccine the week of March 8.
Before people rolled up their sleeve for a shot, the vaccines were reviewed by an advisory group for the Centers for Disease Control and Prevention which had to determine dosage, flag potential side effects and decide who should and shouldn’t get shots. The Pfizer vaccine has been recommended for those 16 and older, prioritizing health care workers and those in nursing homes. The Moderna vaccine is approved for those 18 years and older. The J&J vaccine, formally called the Janssen vaccine, is approved for those 18 years and older.
How Many Doses Of Vaccine Will Wisconsin Get?
That’s up to the federal government. The amount is based on each state’s population.
DHS updates vaccine shipments and the number of people immunized on a regular basis. As of April 28, a total of 4,302,648 doses have been administered. As of April 28, a total of 4,248,455 doses were allocated to Wisconsin, according to state data. The number allocated does not include the retail pharmacy program or other direct federal allocations, according to DHS.
Earlier in the vaccine rollout, Gov. Tony Evers called upon the federal government to send Wisconsin a larger share of the nation’s vaccine supply, and the state requested hundreds of thousands of more doses. It also got vaccinators to speed up the process. On Jan. 15, Evers announced nine mobile labs would be dispatched across the state starting the following week to help with vaccinations. The labs are staffed by members of the National Guard as well as pharmacy and nursing student volunteers through a partnership with the University of Wisconsin System.
On March 18, Evers announced the opening of a new COVID-19 mass vaccination site in Eau Claire on April 8. That’s in addition to four community-based vaccine clinics that will open over the next month. One clinic will be split between Barron and Douglas counties. The others will be located in Racine, La Crosse and Marathon counties. A clinic at Blackhawk Technical College in Rock County opened Feb. 16. Any Wisconsin resident eligible for vaccination may schedule an appointment at any of the clinics.
What Will A Shot Cost?
People won’t be charged for the actual shot when they get vaccinated. Vaccine doses were purchased with U.S. taxpayer dollars. But there will be a bill for administering the shot. Health care providers can charge a person’s private insurance, or public health insurance through Medicare or Medicaid. Insurers are supposed to cover all vaccine fees without customer cost-sharing.
Health systems can also seek reimbursement from the Health Resources and Services Administration’s Provider Relief Fund which was part of the coronavirus relief bill passed by Congress to provide assistance to workers, hospitals and others during the pandemic.
How Many Shots Will You Need?
The J&J vaccine is a single dose. Both the Pfizer and Moderna vaccines require two shots, either 21 or 28 days apart, respectively. The same vaccine must be given for both doses so the medicine matches. People will be reminded when and where to get the second dose from the provider giving the shots. Patients can also check their vaccine schedule on the Wisconsin Immunization Registry.
Vaccinations will be administered by health care systems, local and tribal health departments, nursing homes, assisted living facilities and pharmacies.
Is Wisconsin Ready For A Vaccine?
In late October, DHS released a state COVID-19 vaccination plan outlining a phased-in approach to vaccinating different groups at different times depending on risk and vaccine availability. The following month the state started trying to line up vaccinators to give coronavirus shots.
The Pfizer vaccine needs to be stored at ultra-cold temperatures, colder than -94 degrees Fahrenheit, in special freezers. State officials say each of the state’s seven health care emergency readiness coalition (HERC) regions have facilities where the vaccine can be stored and that they are working with manufacturers of dry ice so the vaccine can be transported. For example, UW Health announced it is serving as a central storage facility for a HERC region’s supply of the Pfizer vaccine.
The J&J vaccine can be stored at refrigerator temperatures for months.

What About Vaccine Safety?
Pfizer enrolled 43,000 people, Moderna enrolled 30,000 people, and J&J enrolled 43,783 people in their clinical trials to come up with evidence on effectiveness and safety, which they present to federal regulators before emergency use authorization is granted.
Outside experts will advise the FDA on whether to approve vaccines and following approval, there will be safety monitoring systems to watch for side effects. The federal government will keep track of any adverse reactions to coronavirus vaccines by relying on providers and the public to report any possible occurrences. Health care workers are required to report to the Vaccine Adverse Event Reporting System.
What About Vaccines For Pregnant And Breastfeeding Women?
Pregnant women in Wisconsin became eligible to receive the coronavirus vaccine as part of Phase 1c, which was eligible March 22.
A study published March 25 in The American Journal of Obstetrics and Gynecology shows the coronavirus vaccines are safe and effective for pregnant and breastfeeding women. The study also showed the vaccines may also offer some protection for their babies. Antibodies were found in breast milk and umbilical cord blood.
The study included 131 participants, according to NPR, vaccinated with either the Moderna or Pfizer vaccine.
Side effects were similar to those who are not pregnant and received the vaccine.
Before studies began to include pregnant and breastfeeding mothers, the CDC said based on how the vaccines work in the body, experts believe they’re unlikely to pose a specific risk for people who are pregnant. Data is limited on the safety of COVID-19 vaccines in pregnant people because none of the initial clinical trials allowed pregnant women to participate — which isn’t uncommon.
Prior to the study’s findings being released, Dr. William Hartman of UW Health told WPR that pregnant women should consider their personal context with their doctor and loved ones. For instance, pregnant or breastfeeding women in groups that are recommended to get the vaccine — like health care workers or essential workers — may decide to get the shots.
There is plenty of precedent, he noted, of pregnant women receiving vaccines safely, like the flu shot.
The CDC notes that with the exception of the smallpox and yellow fever vaccines, “neither inactivated nor live-virus vaccines administered to a lactating woman affect the safety of breastfeeding for women or their infants.”
The vaccines developed by Pfizer and Moderna use an entirely different technology called mRNA that doesn’t contain the virus at all. The J&J vaccine is a viral vector vaccine and does not use mRNA.
“It’s like passing a note into the cell that the cell can produce a protein,” Hartman explained. “And that protein then goes to the cell surface so an antibody can be generated.”
mRNA aren’t thought to pose a risk to the breastfeeding infants whose mothers get the vaccine. In fact, Hartman said the antibodies generated by the vaccine should be able to get into a mother’s breast milk, potentially passing on immunity to an infant.
What About Vaccines For Children?
In January the effects and efficacy of the vaccines began being studied in children. As of May 12, vaccines have been approved for anyone 12 years and older.
On March 31, Pfizer announced its vaccine was 100 percent effective in preventing COVID-19 in children ages 12 to 15, making it so kids in this age group could be eligible for the vaccine before the 2021 school year.
Studies for children younger than 12 are currently underway.
Editor’s note: WPR’s Andrea Anderson, Shamane Mills, Jenny Peek and Bram Sable-Smith contributed reporting to this story.