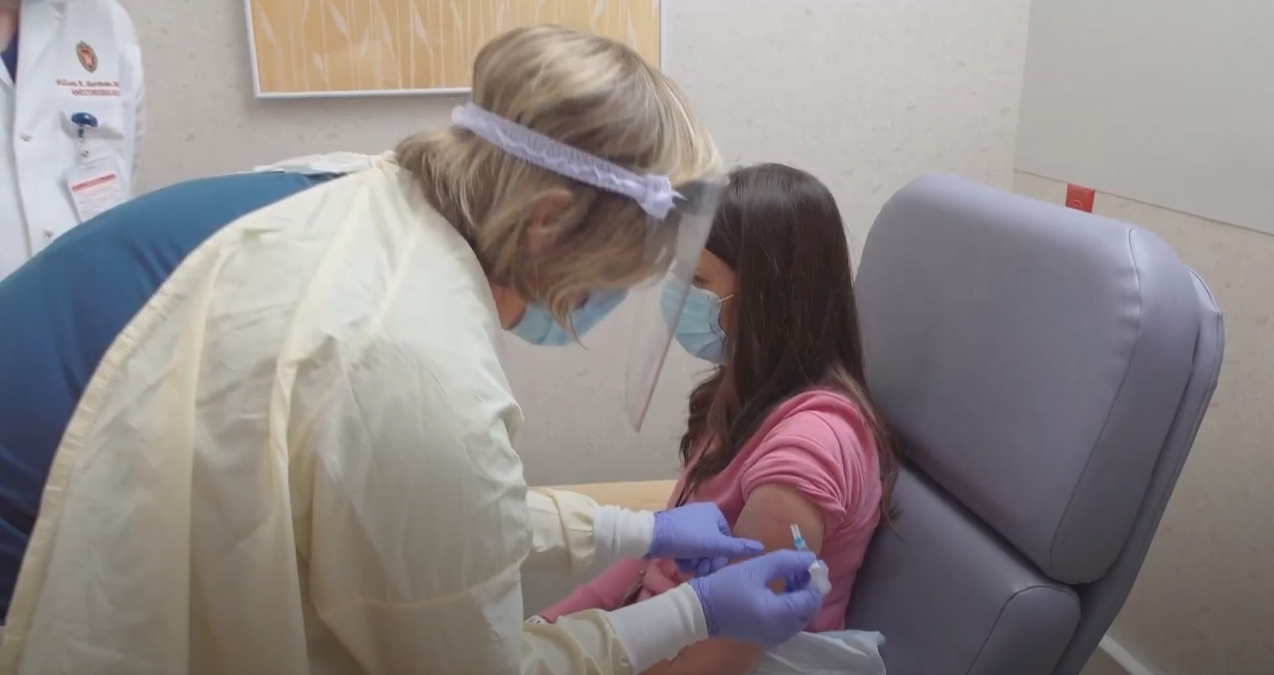
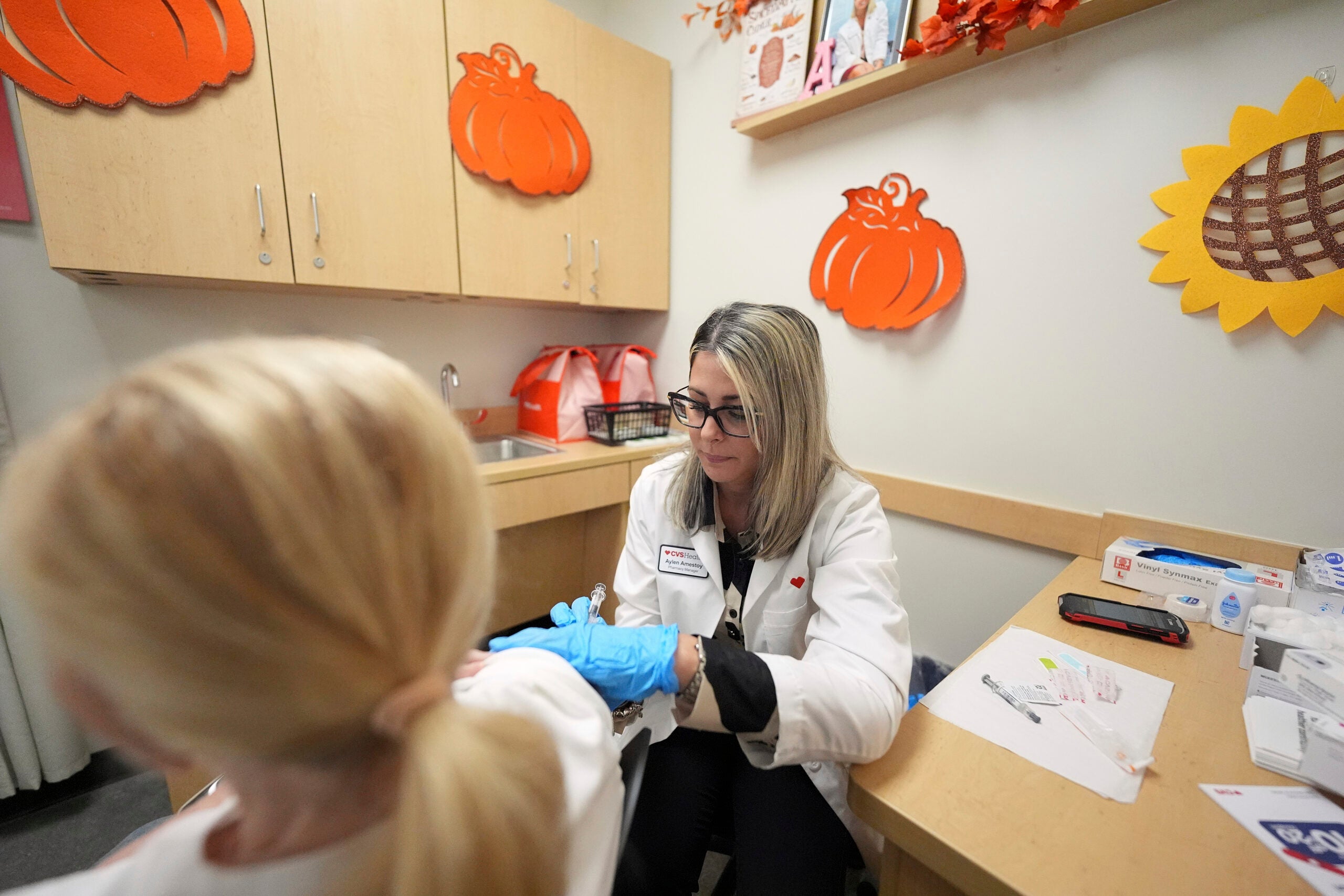
UW Health University Hospital in Madison is one of the test sites for a phase 3 trial of an experimental vaccine for COVID-19, and researchers are trying to recruit a diverse group of people across race, ethnicity, gender and age to participate.
They hope to enroll 1,500 to 2,000 local volunteers for the trial, which is for a vaccine produced by Oxford University and the British pharmaceutical company AstraZeneca. An “aspirational goal” is for 20 percent of those volunteers to be people of color, said UW Health vice president and chief diversity officer Shiva Bidar-Sielaff.
Since COVID-19 has disproportionately affected people of color, she said it’s important to involve people of color in the clinical trials. For example, African Americans make up only 6.7 percent of Wisconsin’s population, but account for 19 percent of the state’s COVID-19 deaths.
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
It’s part of why Bidar-Sielaff decided to participate in the trial herself. She received her first dose of the experimental vaccine last week and will receive another in 30 days.
“I think it’s about making sure there is good representation,” she said. “If you don’t see that people like you participated, it becomes more difficult to trust the outcome of the clinical trials.”
She said it’s important to acknowledge that communities of color sometimes don’t trust clinical trials, due to a well-documented “history of coercion, (a) history of doing clinical trials without appropriate understanding and permission from those who are participating.”
To try to combat any mistrust, researchers are trying to provide as much information as possible to communities of color, said Bidar-Sielaff. Information about the trial in Spanish and Hmong will be available on a website and a phone hotline. Medical interpreters will be provided for more than 24 different languages.
UW Health will also be partnering with local churches, nonprofits and community groups in order to spread the word about the trial and share information about it.
The vaccine is one of three leading candidates being tried in humans for randomized, double-blinded, placebo-controlled clinical trials funded by the U.S. government under Operation Warp Speed. Across the globe, there are 24 coronavirus vaccine candidates currently in clinical trial, according to the World Health Organization.
People who are interested in volunteering for the trial can visit here.
Wisconsin Public Radio, © Copyright 2026, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.