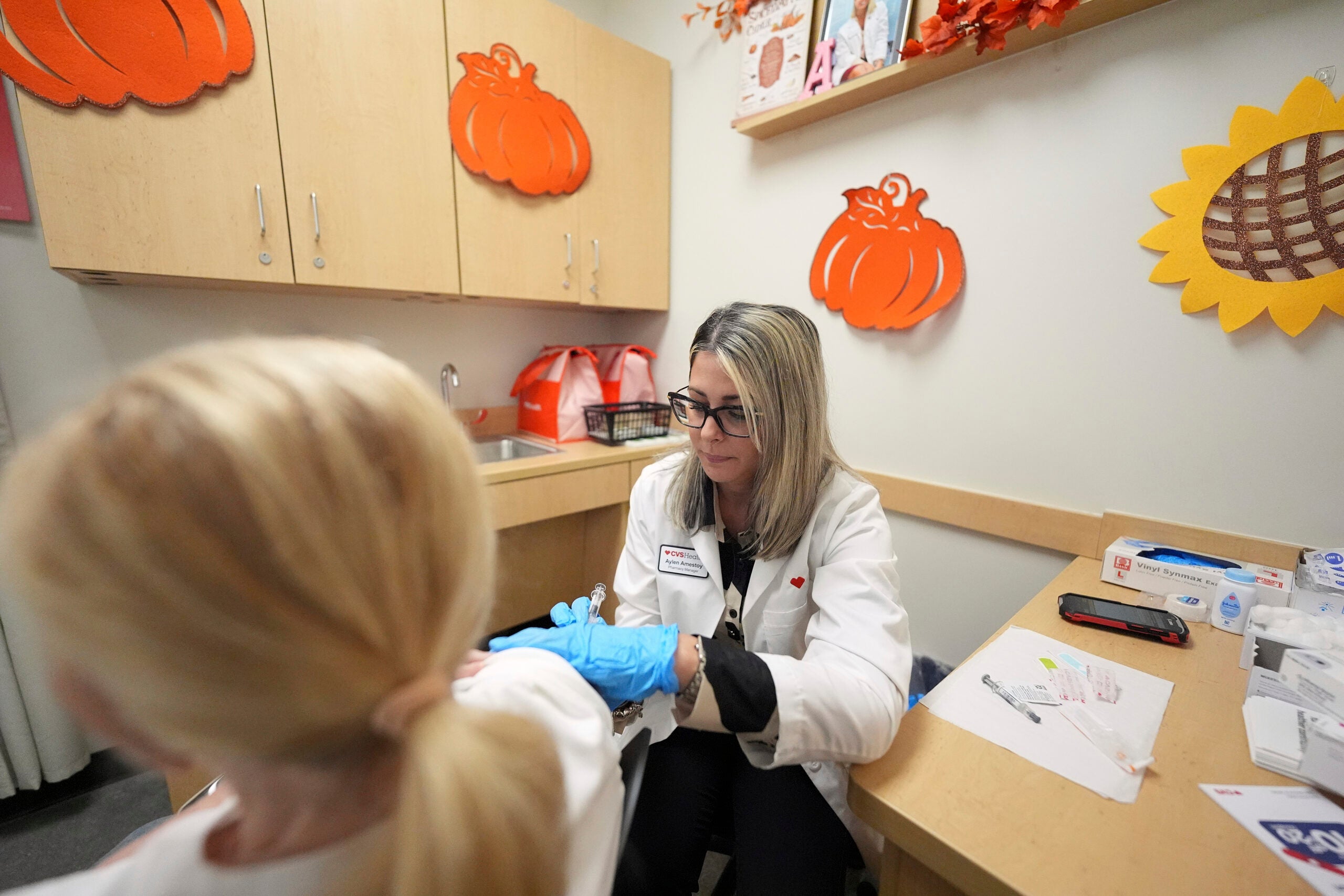
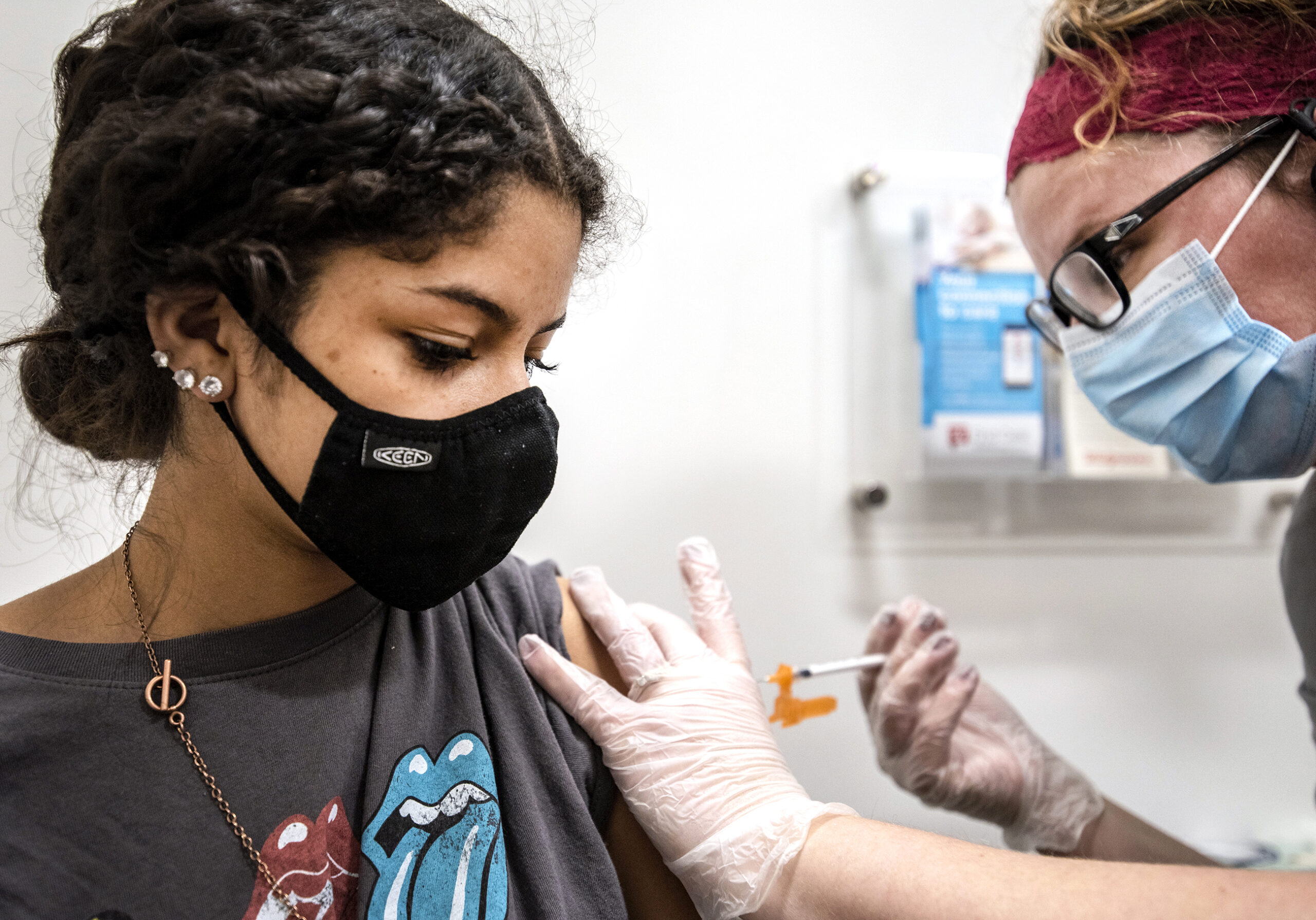
As children ages 5 to 11 begin to get vaccinated against COVID-19, even younger kids are participating in clinical trials that will determine if they will be able to get similar protection against the disease in the near future.
This week, the University of Wisconsin School of Medicine and Public Health announced a phase 3 clinical trial of the Moderna vaccine in kids ages 6 months to 4 years old had filled up. The trial has been underway for two weeks.
“We’ve had an overwhelming response from the Madison community,” said Dr. Bill Hartman. “So, we had way more people interested in the study than we had spots.”
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
Hartman is co-principal investigator of the KidCOVE clinical trial at UW-Madison, which also tested the Moderna vaccine on children ages 5 to 11.
So far, the Pfizer vaccine is the only brand approved for that age group. Moderna could seek emergency use authorization for children ages 5 to 11 next month, Hartman said.
[[{“fid”:”1607411″,”view_mode”:”embed_portrait”,”fields”:{“class”:”media-element file-embed-landscape”,”data-delta”:”1″,”format”:”embed_portrait”,”alignment”:”right”,”field_image_caption[und][0][value]”:”%3Cp%3ETheo%20Rodriguez%2C%204%2C%20chats%20with%20Dr.%20Bill%20Hartman%20of%20UW%20Health%20before%20enrolling%20in%20a%20clinical%20trial%20Nov.%202%20to%20test%20the%20safety%20and%20effectiveness%20of%20the%20Moderna%20COVID-19%20vaccine%20in%20children%20under%20age5%2C%20down%20to%20age%206%20months.%20%3Cem%3EPhoto%20courtesy%20of%20Anne%20Rodriguez%3C%2Fem%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:false,”field_file_image_title_text[und][0][value]”:false},”type”:”media”,”field_deltas”:{“1”:{“class”:”media-element file-embed-landscape”,”data-delta”:”1″,”format”:”embed_portrait”,”alignment”:”right”,”field_image_caption[und][0][value]”:”%3Cp%3ETheo%20Rodriguez%2C%204%2C%20chats%20with%20Dr.%20Bill%20Hartman%20of%20UW%20Health%20before%20enrolling%20in%20a%20clinical%20trial%20Nov.%202%20to%20test%20the%20safety%20and%20effectiveness%20of%20the%20Moderna%20COVID-19%20vaccine%20in%20children%20under%20age5%2C%20down%20to%20age%206%20months.%20%3Cem%3EPhoto%20courtesy%20of%20Anne%20Rodriguez%3C%2Fem%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:false,”field_file_image_title_text[und][0][value]”:false}},”link_text”:false,”attributes”:{“class”:”media-element file-embed-portrait media-wysiwyg-align-right”,”data-delta”:”1″}}]]Dozens of children ages 6 months to 4 years old are enrolled in the hospital’s COVID-19 clinical trial of the Moderna vaccine, according to the medical school. Nationwide, about 2,500 children that age are being given either a placebo, as part of a control group, or COVID-19 vaccine. That dose is one-quarter of the amount approved for adults.
The parents of 4-year-old twins Sam and Theo Rodriguez were eager to participate in the study for the sake of science and to protect their family. The children started school this fall and their grandmother, who has diabetes and COPD, lives with them at their home in eastern Dane County.
No one in the family has ever participated in clinical trial before, said their mother, Anne Rodriguez. But she said they were watching for this opportunity in hopes of potentially getting early access to a vaccine for their young children.
Hartman expects the Moderna clinical trial in these younger children to be finished in mid-December. Pfizer is further along in testing its vaccine on children under age 5, but federal approval isn’t expected until 2022.
Wisconsin Public Radio, © Copyright 2026, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.