As American and U.K. regulators approve the world’s first gene-editing treatment for sickle cell disease, Wisconsin scientists are researching how to use the same technology to treat two eye diseases that cause blindness.
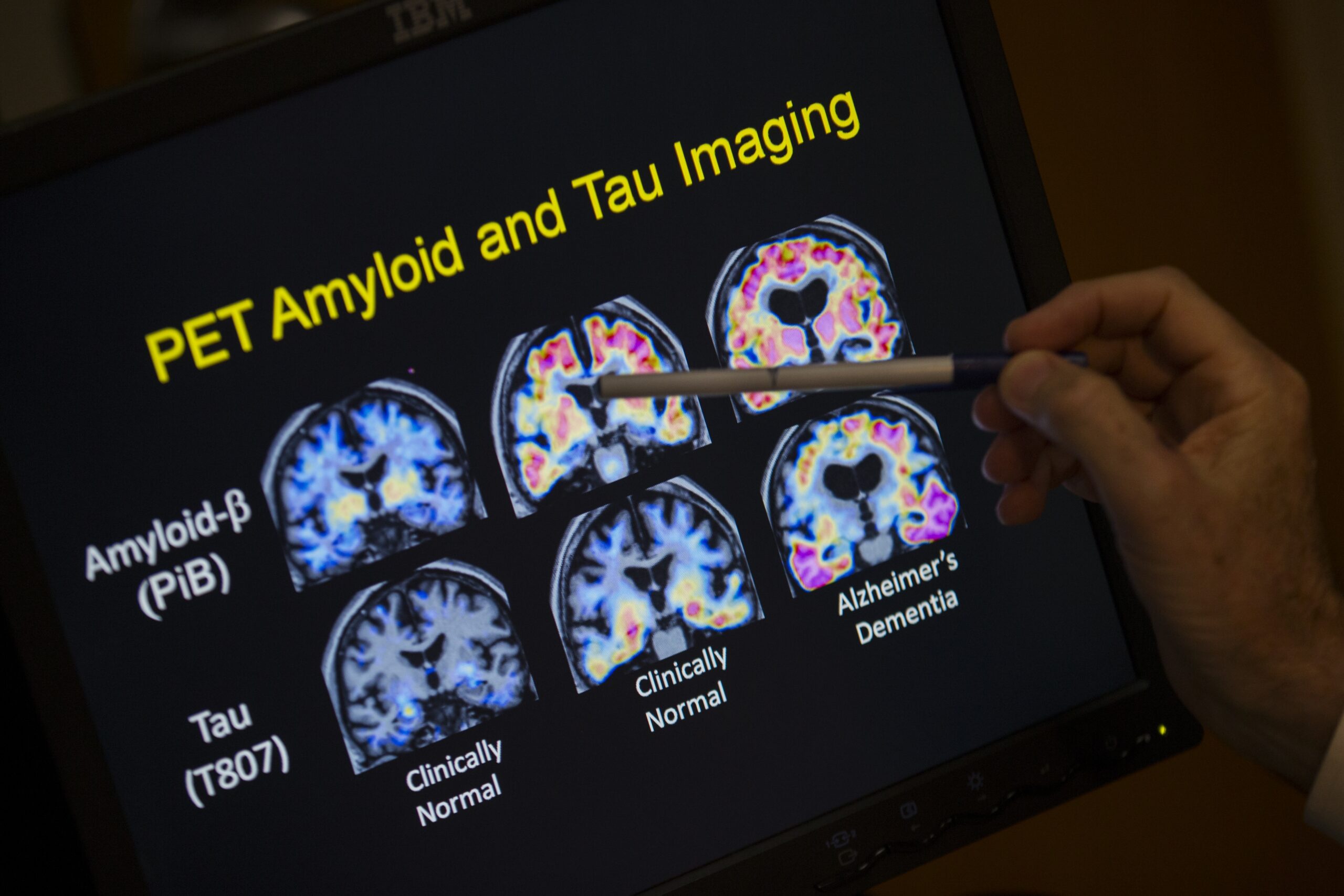
Krishanu Saha leads the CRISPR Vision Program at the University of Wisconsin-Madison and is member of National Institute of Health’s Somatic Cell Genome Editing Consortium. His lab is specifically studying how to cure Best disease as well as Leber congenital amaurosis, one of the most common causes of blindness in children.
“All of the testing that we’ve done thus far shows a lot of promise that it can actually correct the defects in these cells. And so the task for us over the next five years is to formulate a medicine that could be used here in trials enrolling patients,” Saha said in a recent interview with WPR’s “The Morning Show.”
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
CRISPR is a ground-breaking technology that allows scientists to modify genetic code. In 2020, its creators won the Nobel Prize in Chemistry. Research using CRISPR is now underway globally to treat high cholesterol, cystic fibrosis, muscular dystrophy and other illnesses.

The following was edited for clarity and brevity.
Kate Archer Kent: United Kingdom regulators first approved the gene-editing therapy CRISPR in November to treat sickle cell disease. How significant is this milestone in medicine?
Krishanu Saha: Well, it’s the first. I think the entire field is just elated to hear this news. I would say about 10 years ago, when the first discoveries of CRISPR came out, it was an abstract idea that this could actually be used in a medicine and prescribed by doctors.
KAK: Could you explain how gene-editing therapy works and maybe in the context of sickle cell disease, for instance?
KS: I’ll back up just a second to say that CRISPR is a tool that can specifically change letters in the genetic code. We have the human genome within ourselves. We have a copy from each of our parents. So we have two copies of 3 billion letters in our code.
What’s remarkable is that CRISPR can go into this code and change just one letter and just one letter can unfortunately cause disease. In the case of sickle cell, which is one of these examples, a letter change on both your copies of the genome can lead to the sickle cell trait causing this sickle shape of your red blood cells.
KAK: CRISPR uses a viral delivery system to edit genes in most cases. Are there downsides to this method?
KS: The drawbacks to using viruses is that our bodies can recognize them as foreign, and so after one-time treatment, most patients develop antibodies against the virus such that if you wanted to reduce and give more of the drug, it is not very effective. Your body can recognize it and create an immune response.
One of our strategies that we’re developing is to avoid viruses altogether. We use some new chemical compounds that are in nanoparticles that can essentially mask the CRISPR machinery… Those are really exciting approaches developed by my lab and our collaborators here that we’re trying to move into the clinic here in Wisconsin within five years or so.
KAK: The CRISPR Vision Program at UW-Madison researches treatments for two hereditary diseases that cause blindness — Best disease and Leber congenital amaurosis. Why focus your research on these eye diseases?
KS: The promise of CRISPR is to have a one-shot cure, and in these cases, the genetic mutation is only a small stretch of letters (in genetic code)… The task for us over the next five years is to formulate a medicine that could be used here in trials enrolling patients. We think the eye is an accessible organ, because we can deliver-inject through these routes of administration easily right next to the cell types that are affected in these diseases.
There’s a number of technical reasons why we think the delivery challenges are manageable, and plus, the amount of material that you need to have to modify the retina is relatively small compared to a systemic disease like muscular dystrophy where you need to fix arguably all the muscles in the body. The portion of the retina is really only a few millimeters, arguably, that you use for vision that leads to a lot of meaningful functional benefits.
KAK: Where is your research headed next?
KS: We’re developing some exciting applications of CRISPR in the cancer therapy space. We’re taking out T-cells and natural killer cells and modifying them such that they can detect cancer. CRISPR is an amazing tool to be able to write in the type of functionality that we want to personalize cancer care.
Wisconsin Public Radio, © Copyright 2026, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.