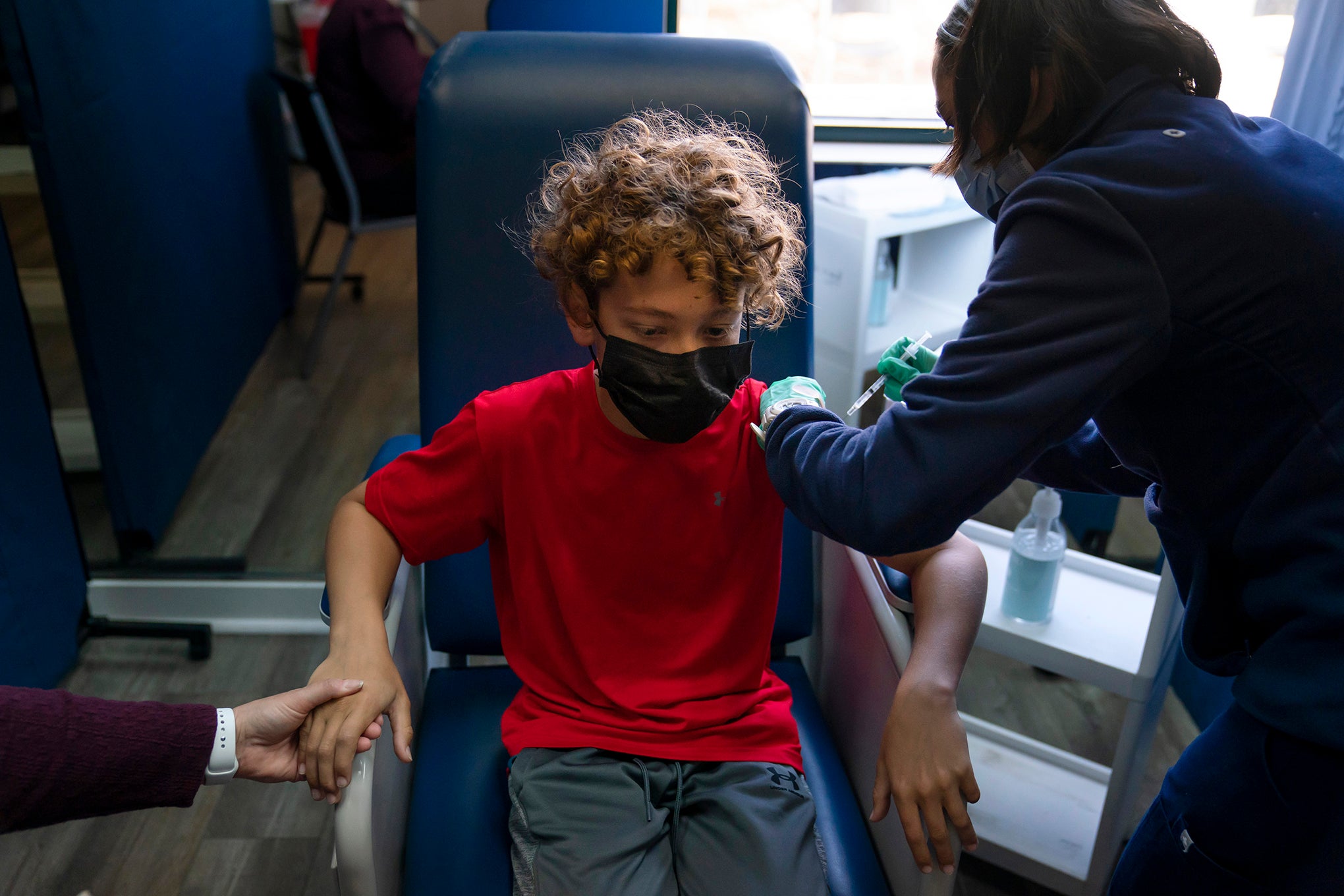
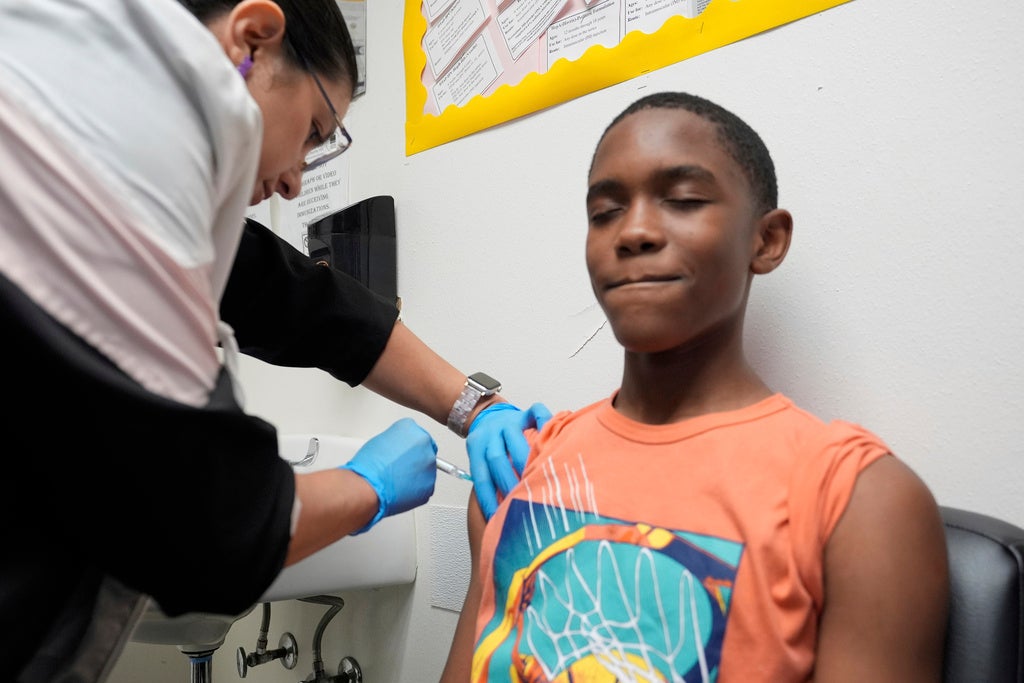
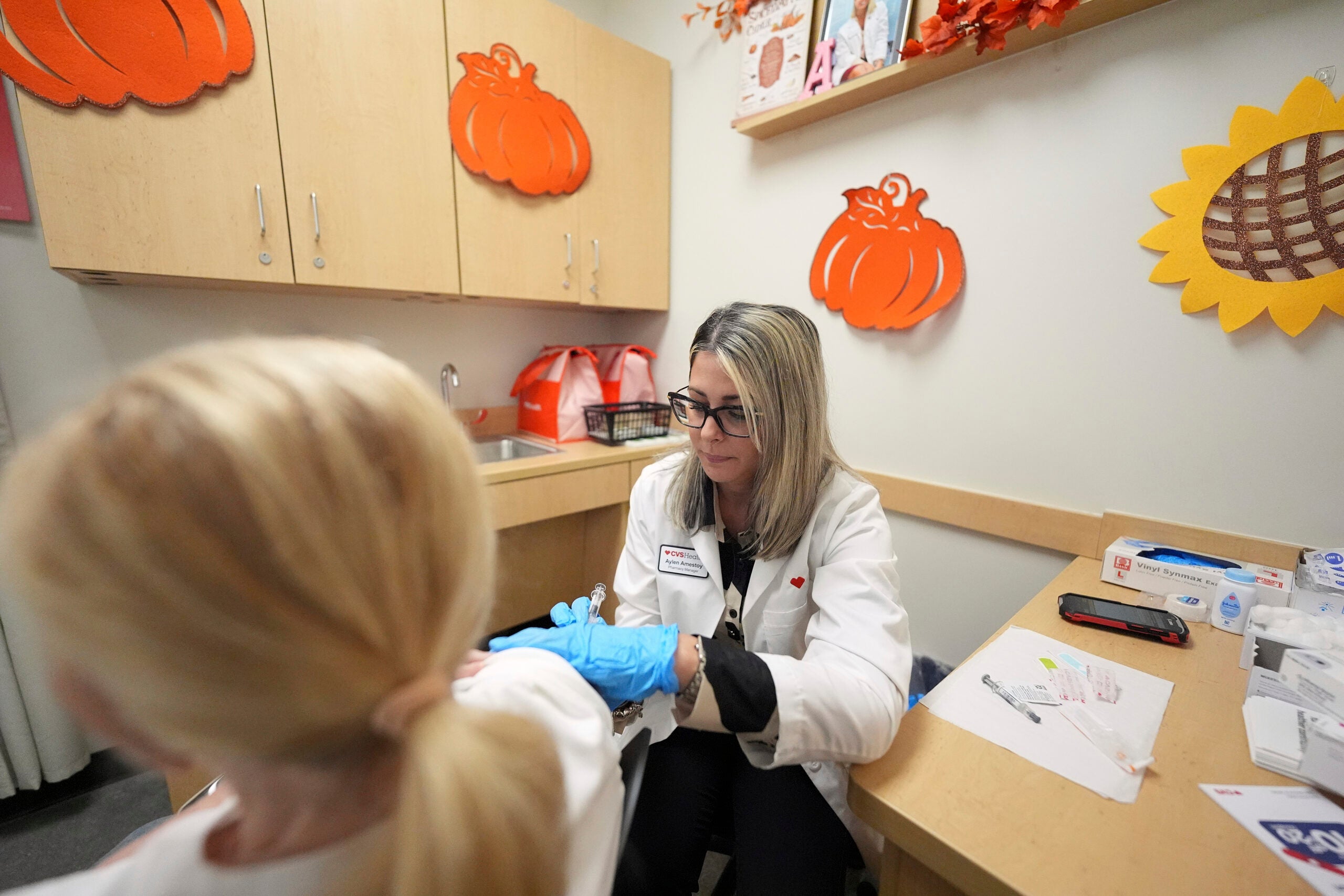
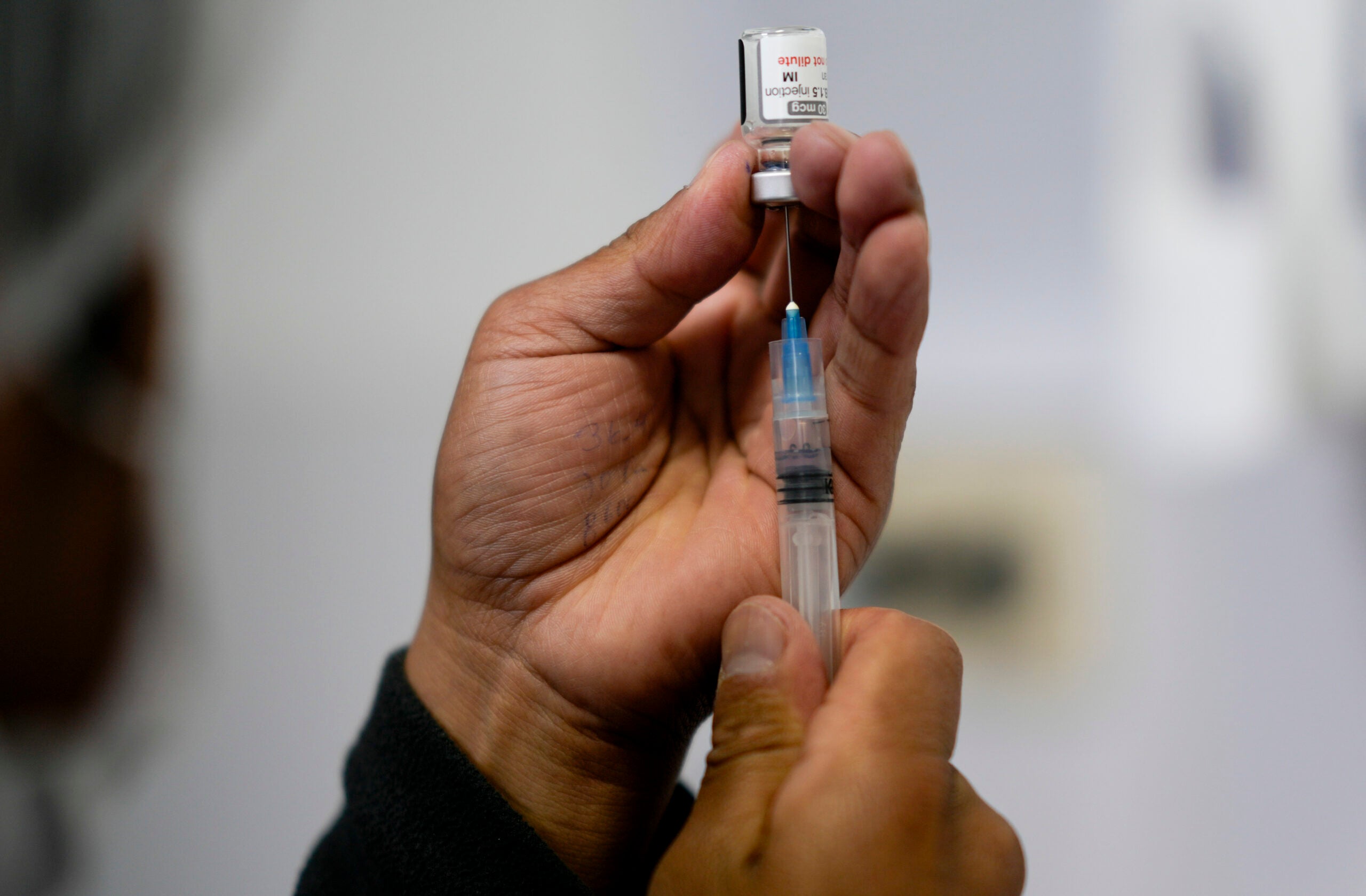
UW Health will be participating in a clinical trial of the Moderna vaccine for the remaining group of children not yet eligible to get a shot. On Friday, enrollment will begin for children 6 months to 11 years old. The 14-month study will evaluate the safety and effectiveness of the Moderna COVID-19 vaccine.
Eighty children will be enrolled at American Family Children’s Hospital in Madison. The University of Wisconsin-Madison School of Medicine and Public Health is part of a larger Moderna study taking place at more than 75 locations in the United States and Canada, which aims to have as many as 12,000 children taking part.
Children will get two doses of the COVID-19 vaccine, four weeks apart. Older children ages 6 to 11 will be studied first, then those ages 2 to 6 years old, and finally the youngest group of children age 6 months to 2 years old.
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
The first group to be studied — kids ages 6 to 11 — will get half the dose adults get said Dr. William Hartman, a UW Health anesthesiologist and co-leader of the study.
Study sites around the country will be administering different levels of vaccine to see what is the minimum amount that is most effective to protect against the disease, he said. Not everyone will get the Moderna vaccine; 1 in 3 participants will get a placebo.
Children in the study will be monitored closely for adverse effects. Some adolescents and young adults have experienced heart swelling, or myocarditis, after getting the Pfizer and Moderna vaccines. The condition is rare and the Centers for Disease Control and Prevention is monitoring these reports.
“The vaccines themselves induce an inflammatory state. That’s what you want them to do is make antibodies that go after the virus,” said Hartman. “Sometimes this will put the body into a hyperimmune state where the body has a big inflammatory response which is likely leading to myocarditis.”
Currently, only the Pfizer vaccine is available for children 12 and older. Moderna has also applied to the U.S. Food and Drug Administration for approval of its vaccine for the same age group.
In the meantime, Dane County Public Health Director Janel Heinrich says parents can protect their unvaccinated kids by choosing where they go and whether they mask up.
“Someone who has a child under 12 in their household who is not eligible to receive the vaccine might make more cautious decisions than someone whose entire household is vaccinated,” she said during a Thursday media briefing.
Federal health officials have recommended everyone wear masks indoors — regardless of vaccination status — in COVID-19 hotspots.
Those interested in signing up their child for the COVID-19 clinical trail can go to www.kidcovestudy.com or call 608-262-8300.
Wisconsin Public Radio, © Copyright 2026, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.