What kind of futures are America’s elite schools preparing their students for? We speak with a former Yale professor who says these schools aren’t actually leading students to productive and meaningful lives. Then we learn more about the process of tax inversion, and hear about the debate over a drug the helps prevent the transmission of HIV.
Featured in this Show
-
Former Yale Professor Takes A Critical Look At The Country's Best Colleges
Many high-achieving high school students dream of one day attending one of the Ivy League or equivalent colleges. A former Yale professor offers a critical look at the elite college system in the United States and discusses an alternative way to a more meaningful life.
-
Burger King Deal Shines Spotlight On Tax Inversion
The aquisition of the Canada-based restaurant chain Tim Horton’s by Burger King is shining the spotlight on a process known as tax inversion. An economics experts talks about how tax inversion works, why it seems to be becoming more popular, and the impact it could be having on the U.S. economy.
-
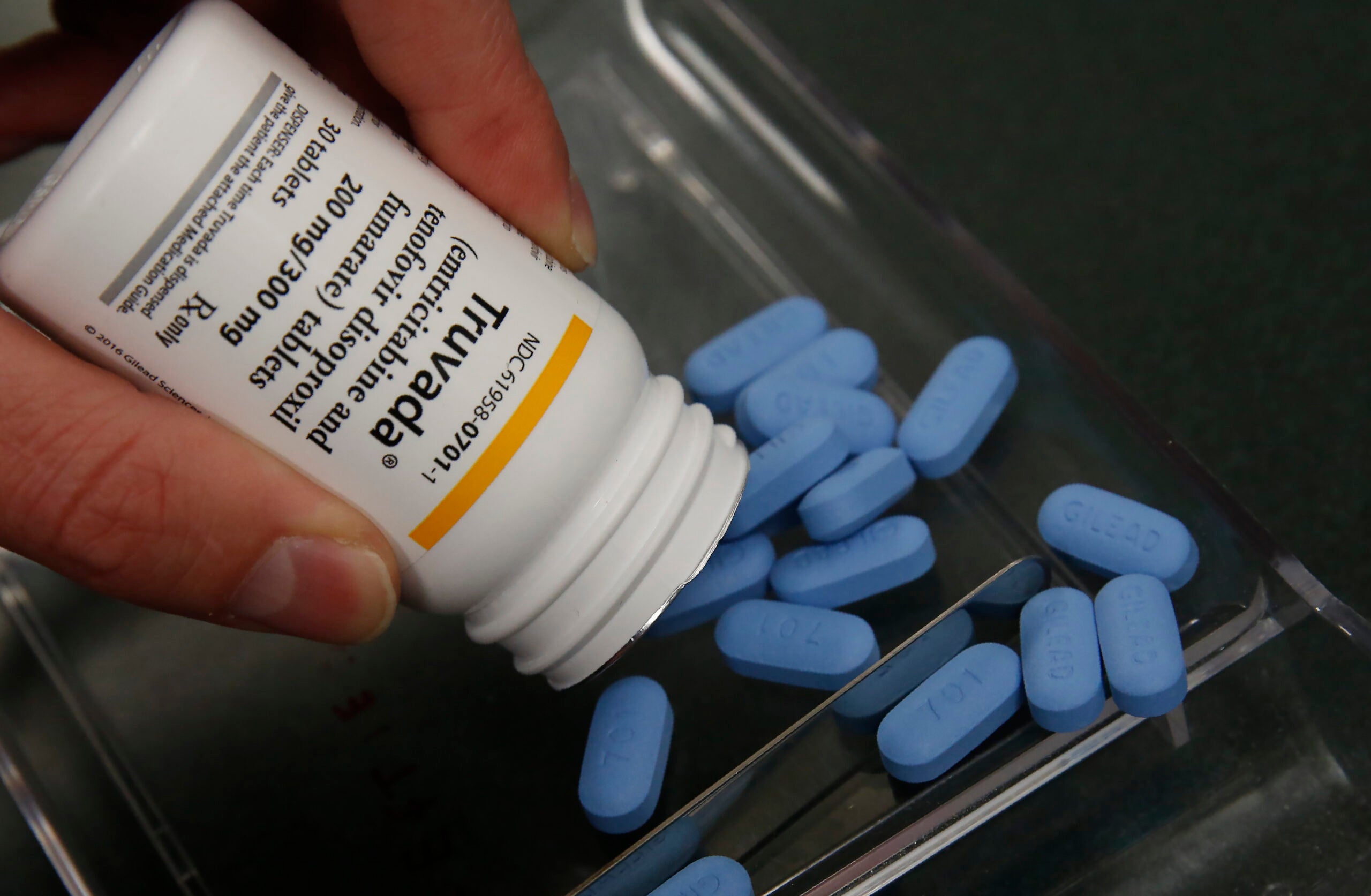
Wisconsin AIDS Resource Center Weighs In On Truvada, Drug That Helps Prevent HIV
Since the AIDS epidemic began in the early 80s, doctors the world over have been trying to find ways to cure and prevent the disease. In 2012, three decades after the official start of the epidemic, the FDA approved the first drug ever to help prevent HIV for people at a high risk of transmission. It’s called Truvada, also known as PrEP, and is said to be over 90% effective.
Two years after the drug’s release on the market, its uptake has been slow and it’s been the subject of some debate within the HIV prevention and gay communities. The Director of Clinical Pharmacy at the AIDS Resource Center of Wisconsin weighs in.
Episode Credits
- Rob Ferrett Host
- Veronica Rueckert Host
- Amanda Magnus Producer
- Chris Malina Producer
- Galen Druke Producer
- William Deresiewicz Guest
- Chris Farrell Guest
- Nick Olson Guest
Wisconsin Public Radio, © Copyright 2024, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.