As the number of available intensive care unit beds drops to near zero in many regions of Wisconsin, doctors with the Mayo Clinic Health System say they’re hopeful new oral, antiviral pills seeking federal approval could change the trajectory of the COVID-19 pandemic.
During a media briefing Monday, top doctors with Mayo said their ability to use therapeutic treatments for hospitalized COVID-19 patients is improving.
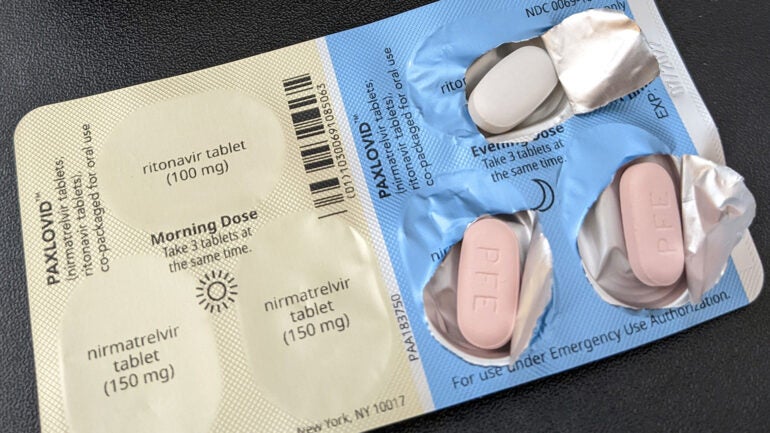
Dr. Andrew Badley is a professor of infectious disease and chair of the health system’s COVID-19 task force. He said new oral therapies, seeking authorization by the Food and Drug Administration, known as Paxlovid and Molnupiravir are showing great promise in clinical trials, though there are concerns about the safety of molnopiravir in regard to pregnancy.
Stay informed on the latest news
Sign up for WPR’s email newsletter.
Badley said Paxlovid, which was developed by Pfizer, targets an enzyme in the virus at the molecular level that causes COVID-19 and disrupts its ability to grow and replicate itself.
“It has an almost 90 percent — 89 percent — ability to prevent hospitalization and death in those at high risk,” said Badley.
The FDA is still considering an authorization for Paxlovid.
Badley said Molnupiravir, which was developed by drug makers Merck and Ridgeback, has shown a 30 percent reduction in the risk of COVID-19 hospitalization or death. An FDA advisory panel voted narrowly in favor for an emergency use authorization for the drug Nov. 30.
Dr. Raymond Razonable is a professor of medicine at Mayo Clinic in Rochester, Minnesota, and vice chair for the health system’s division of infectious diseases. Razonable has led Mayo’s monoclonal antibody treatment program.
“Our program has infused almost 20,000 infusions of monoclonal antibodies since we started in November 2020, and we have seen remarkable outcomes in terms of it reducing the risk of hospitalization and progression to severe disease,” said Razonable.
Badley said the oral, antiviral drugs seeking FDA approval could be game changing for hospitals because they don’t need to be administered intravenously and wouldn’t need the same cold storage requirements as some of the current monoclonal antibody drugs.
He said one scientific issue that needs to be understood before the antiviral pills are rolled out for widespread use is whether they might cause the coronavirus to mutate and become more resistant to the therapies. Badleys said that hasn’t happened with COVID-19, “possibly because treatment courses are short.”
“I think there are huge opportunities for these oral antivirals, which I call first-generation oral antivirals, as well as future-generation oral antivirals to really change the trajectory of the pandemic,” said Badley.
Badley and Razonable were asked whether the emerging treatments would be effective against the newly discovered omicron variant of COVID-19. They said more clinical evidence is needed, but they suspect medications that don’t target the virus’s spike protein will be effective. The two also said vaccination against COVID-19 will likely be one of the main protections against omicron, along with wearing masks indoors — regardless of vaccination status.
As the FDA reviews new treatment options for COVID-19, Wisconsin hospitals are dealing with a surge of people in need of treatment.
According to the Wisconsin Hospital Association COVID-19 dashboard, there were 1,562 people in the state hospitalized for COVID-19 as of Monday afternoon. That’s an increase of 130 over the past week. There were 407 COVID-19 patients in intensive care units across the state Monday. Hospitals in the Fox Valley region reported having two intensive care unit beds available. North Central region hospitals reported one available ICU bed. There were three ICU beds available in Northwest Wisconsin and zero ICU beds available in western Wisconsin.
As of Monday, a New York Times ranking of states and United States territories with the highest increase of viral transmission ranks Wisconsin fifth in the nation, with a 20 percent increase in cases over the last two weeks.
The Wisconsin Department of Health Services reported 3,548 new confirmed cases of COVID-19 as of Dec. 2 — the last day DHS updated their coronavirus dashboard — and the seven-day average of new cases was 3,548 as of Dec. 2
Wisconsin Public Radio, © Copyright 2024, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.